Upstream / Downstream
Explore pathways related to this product.
Research More. Spend Less.
Spend $650 or more and get 20% off*
(*Offer valid in the US only. Expires June 30, 2017)
To Purchase # 7341S
| 7341S | 500 µl (250 PCR reactions) | $102.00 |
| $0.00 | ||
SimpleChIP® Mouse HoxA1 Promoter Primers #7341
SimpleChIP® Mouse HoxA1 Promoter Primers were tested on DNA isolated from cross-linked cells using the SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. Real-time PCR was performed in duplicate on a serial dilution of 2% total input DNA (20 ng, 4 ng, 0.8 ng, and 0.16 ng) using a real-time PCR detection system and SYBR® Green reaction mix. The PCR amplification efficiency (E) and correlation coefficient (R2) were calculated based on the corresponding threshold cycle (CT) of each dilution sample during 40 cycles of real-time PCR (95°C denaturation for 15 sec, 65°C anneal/extension for 60 sec).
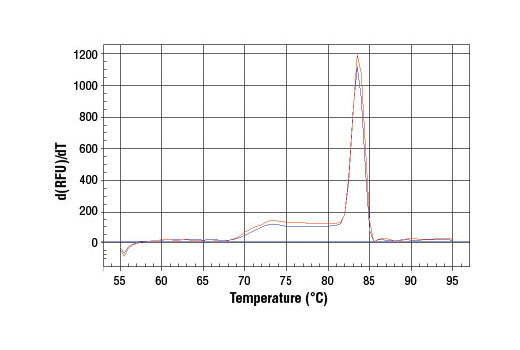
Learn more about how we get our imagesPCR product melting curves were obtained for real-time PCR reactions performed using SimpleChIP® Mouse HoxA1 Promoter Primers. Data is shown for both duplicate PCR reactions using 20 ng of total DNA. The melt curve consists of 80 melt cycles, starting at 55°C with increments of 0.5°C per cycle. Each peak is formed from the degradation of a single PCR product.
Learn more about how we get our imagesProduct Description
SimpleChIP® Mouse HoxA1 Promoter Primers contain a mix of forward and reverse PCR primers that are specific to a region of the mouse Homeobox A1 (HoxA1) promoter. These primers can be used to amplify DNA that has been isolated using chromatin immunoprecipitation (ChIP). Primers have been optimized for use in SYBR® Green quantitative real-time PCR and have been tested in conjunction with SimpleChIP® Enzymatic Chromatin IP Kits #9002 and #9003 and ChIP-validated antibodies from Cell Signaling Technology®. The homeobox gene clusters are silenced by members of the polycomb family.
Specificity / Sensitivity
Background
The chromatin immunoprecipitation (ChIP) assay is a powerful and versatile technique used for probing protein-DNA interactions within the natural chromatin context of the cell (1,2). This assay can be used to either identify multiple proteins associated with a specific region of the genome or to identify the many regions of the genome bound by a particular protein (3-6). ChIP can be used to determine the specific order of recruitment of various proteins to a gene promoter or to "measure" the relative amount of a particular histone modification across an entire gene locus (3,4). In addition to histone proteins, the ChIP assay can be used to analyze binding of transcription factors and co-factors, DNA replication factors, and DNA repair proteins. When performing the ChIP assay, cells are first fixed with formaldehyde, a reversible protein-DNA cross-linking agent that "preserves" the protein-DNA interactions occurring in the cell (1,2). Cells are lysed and chromatin is harvested and fragmented using either sonication or enzymatic digestion. Fragmented chromatin is then immunoprecipitated with antibodies specific to a particular protein or histone modification. Any DNA sequences that are associated with the protein or histone modification of interest will co-precipitate as part of the cross-linked chromatin complex and the relative amount of that DNA sequence will be enriched by the immunoselection process. After immunoprecipitation, the protein-DNA cross-links are reversed and the DNA is purified. Standard PCR or quantitative real-time PCR are often used to measure the amount of enrichment of a particular DNA sequence by a protein-specific immunoprecipitation (1,2). Alternatively, the ChIP assay can be combined with genomic tiling micro-array (ChIP on chip) techniques, high throughput sequencing (ChIP-Seq), or cloning strategies, all of which allow for genome-wide analysis of protein-DNA interactions and histone modifications (5-8). SimpleChIP® primers have been optimized for amplification of ChIP-isolated DNA using real-time quantitative PCR and provide important positive and negative controls that can be used to confirm a successful ChIP experiment.
1. Orlando, V. (2000) Trends Biochem Sci 25, 99-104.
2. Kuo, M.H. and Allis, C.D. (1999) Methods 19, 425-33.
3. Agalioti, T. et al. (2000) Cell 103, 667-78.
4. Soutoglou, E. and Talianidis, I. (2002) Science 295, 1901-4.
5. Mikkelsen, T.S. et al. (2007) Nature 448, 553-60.
6. Lee, T.I. et al. (2006) Cell 125, 301-13.
7. Weinmann, A.S. and Farnham, P.J. (2002) Methods 26, 37-47.
8. Wells, J. and Farnham, P.J. (2002) Methods 26, 48-56.
Data Sheets & Documentation
For Research Use Only. Not For Use In Diagnostic Procedures. Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc. SimpleChIP is a registered trademark of Cell Signaling Technology, Inc. SYBR is a registered trademark of Life Technologies Corporation.